
Breast implant-associated anaplastic large cell lymphoma (BIA-ALCL): a collaborative effort for diagnosis and treatment
Introduction
Breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) is a rare clinical entity. As of September 30, 2017, the Food and Drug Administration (FDA) has received 414 reported cases worldwide with 9 deaths. BIA-ALCL is classified as a non-Hodgkin lymphoma (NHL). NHLs of the breast account for 0.01–0.5% of breast cancers and within this subgroup of cancers, most of the occurrences are B-cell in origin. The primary treatment modality of these B-cell lymphomas is chemotherapy. Unlike the B-cell originating cancers, BIA-ALCL is T-cell in origin and is usually curable with complete surgical excision of the implant and capsule. BIA-ALCL typically presents 2–28 years after implant placement as a fluid collection around the implant or as a mass associated with the implant capsule. Approximately 550,000 breast implants are placed annually in the US and an estimated 10 million women worldwide have breast implants. In the US, about 12.7% [70,000] of the implants placed are textured and this has been stable over the past 6 years. It is imperative to educate patients about this rare but often curable cancer. Additionally, we believe the early multidisciplinary involvement of both general and plastic surgeons is of the utmost importance when approaching patients with BIA-ALCL.
Case presentation
A 37-year-old female presented to an outpatient plastic surgery clinic in January 2008 (Figure 1). The patient complained of a two-year history of fluctuation in size of the left breast. This patient had a history of cosmetic bilateral augmentation mammoplasty with saline Biocell textured implants in 1992. Family history of breast cancer was absent and screening mammography in 2007 demonstrated a normal left breast. On initial presentation to the plastic surgeon, the patient was diagnosed with a presumed seroma. Incision and drainage of the fluid demonstrated atypical cells showing high nucleus to cytoplasm ratio, identified as “exaggerated macrophages” in the pathology report. The patient’s symptoms appeared to resolve after drainage of the seroma.
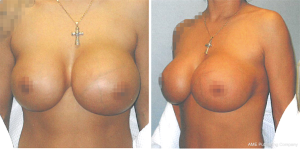
In April 2015, our patient underwent diagnostic mammography for a self-discovered mass of the left breast. The radiologic report indicated a BI-RADS 4A suspicious abnormality with 2–10% chance of malignancy. The appearance was described by the radiologist as an infected fluid collection surrounding the left breast implant suspicious for abscess with mastitis and reactive lymphadenopathy. Ultrasound-guided aspiration was attempted but failed to aspirate fluid from the lateral margin of the left breast implant due to extensive septation of the collection. In August 2015, our patient presented to surgical oncology at our hospital with pain and a new palpable mass in the left breast. Surgical excision produced a 12 cm × 12 cm × 6 cm, 151-gram irregular specimen with pale tan to opaque yellow tissue. Immunohistochemical (IHC) staining demonstrated positivity for CD30, indicating a high likelihood for lymphoma, with focal positivity for the T-cell marker CD43 in tumor cells. IHC staining was negative for ALK-1, Bol-2, EBER, Pax5, CD2, CD3, CD4, CD5, CD10, CD20, and CD79a (Table 1).
Table 1
| Specimen | Positive | Negative |
|---|---|---|
| Initial excisional biopsy | CD25, CD30, CD43 (focal), HUM-1 | ALK-1, pan-cytokeratin, CK7, Melan A, Bol-2, EBER, Pax5, CD2, CD3, CD4, CD5, CD10, CD20, CD79a |
| Subsequent capsulectomy | CD30, CD45, granzyme B, perforin | ALK-1, CD3, CD4, CD43 |
In October 2015, our patient was evaluated by PET/CT imaging which demonstrated localized disease to the soft tissue encasing the left breast implant. Medical oncology recommended surgical intervention without chemotherapy. In December 2015, a left breast partial capsulectomy with implant removal was performed by surgical oncology. Pathology identified malignant cells with positive staining for CD30, CD45, granzyme B, and perforin (Table 1). Microscopic examination identified extensive multifocal necrosis of a large cell lymphoma with hypercellularity of large lymphoid cells. Nuclei were reniform, horse-shoe, and dough-nut shaped with multiple nucleoli and abundant eosinophilic cytoplasm. These findings were consistent with a T-cell anaplastic large cell lymphoma (T-ALCL) associated with the breast capsule.
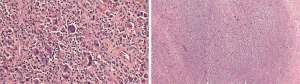
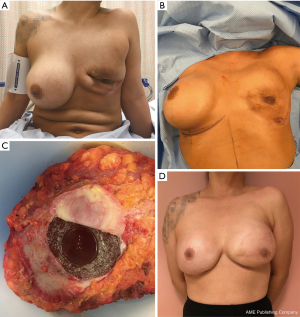
By March 2016, palpable local recurrence was evident and repeat PET/CT showed invasion of local lymph nodes. Medical oncology-initiated chemotherapy with cytoxan, adriamycin, vincristine, and prednisone (CHOP) for four treatment cycles. In May 2016 a joint surgical procedure with surgical oncology and plastic surgery performed left nipple-sparing mastectomy with total capsulectomy and right implant removal with capsulectomy. Pathology demonstrated recurrent T-ALCL of the left breast (Figure 2) with negative margins and a negative sentinel lymph node. The right breast capsule was negative for malignancy. Following completion of CHOP chemotherapy, the patient underwent left latissimus dorsi myocutaneous flap reconstruction with tissue expander placement, followed by bilateral implant placement. Currently the patient remains in remission for 2 years (Figure 3).
Discussion
Anaplastic large cell lymphoma (ALCL) is an uncommon form of NHL accounting for only 3% of all NHL. The association of ALCL with breast implants is even rarer with only 414 total reports filed as of September 30, 2017, including 9 deaths. There appears to be a positive association with textured implants versus smooth implants with 384 out of the 414 total reported implant surfaces being textured. The FDA specifically notes that the smooth implant reports were either mixed implant case histories or that no clinical history was provided. There is minimal association with implant fill material (silicone 56% versus saline 43%) and reason for prosthetic implantation (reconstruction 14% versus augmentation 21%), although the reason for implantation was not specified for 65% of patients. Common clinical presentations in descending order include seroma, breast swelling/pain, capsular contracture, and peri-implant mass (1).
The two clinical entities of BIA-ALCL include effusion-associated ‘in-situ’ BIA-ALCL and ‘infiltrative’ mass-forming BIA-ALCL. Effusion-associated BIA-ALCL usually presents as a seroma with malignant cells confined to the serous fluid and capsule surface. This non-invasive pattern suggests a more favorable prognosis with an indolent course, responding to capsulectomy and implant removal alone. The breast-mass producing variant histologically shows diffuse infiltration of malignant cells into the capsule and adjacent tissues. This infiltrative form has a poor prognosis with an aggressive course requiring chemotherapy and/or radiation therapy (1). Seroma formation is commonly seen in the infiltrative form, as observed in our patient, and must not impede further workup for invasive disease.
The strongest cytologic features for identification of this cancer are positive CD30 and negative anaplastic lymphoma kinase-1 (ALK-1) on IHC staining. CD30 belongs to the tumor necrosis factor (TNF) family of cell surface receptors and acts as an activation antigen for lymphocytes. Overexpression is implicated in the molecular pathogenesis of aberrant growth and cytokine expression in lymphoma. ALK-1 negativity is a common finding in primary breast implant associated ALCL versus ALK-1 positivity which is found in systemic ALCL (2). Ultrasound-guided aspiration of seroma or surgical biopsy of capsule and seroma should be performed and sent for IHC staining to confirm a suspected diagnosis of BIA-ALCL. Following diagnosis, a PET/CT should be performed to rule out regional or systemic spread. The National Comprehensive Cancer Network describes capsulectomy and implant removal as optimal treatment for disease confined to the capsule and with resectable mass. Advanced disease including metastasis to local lymph nodes or organ metastasis requires chemotherapy.
It is important to involve a plastic surgeon early in the management of abnormal findings associated with breast implants. The appearance of the capsule with tumor burden may appear similar to that of a benign capsule. Had plastic surgery been involved at the time of initial capsulectomy, our patient may have undergone a more extensive capsulectomy, thereby preventing recurrence, repeat operation and the need for systemic chemotherapy. A study investigating treatment outcomes for BIA-ALCL found that complete surgical excision significantly improved overall survival time and event-free survival in both mass and effusion localized disease (3). The standard chemotherapy regimen recommended is a CHOP anthracycline-based protocol. Currently, neoadjuvant treatment with brentuximab vedotin is under investigation for CHOP-resistant BIA-ALCL. Brentuximab vedotin is a monoclonal antibody drug-conjugate selectively targeting the CD30 antigen (4). Radiotherapy is reserved for local unresectable disease.
Complete surgical excision for treatment of BIA-ALCL includes removal of the implant, total capsulectomy, and complete removal of any tumor with negative margins. Determination of the need for concomitant mastectomy should be made based on the presence and extent of local or regional tumor invasion, although more research is required to investigate the value of mastectomy following diagnosis of BIA-ALCL. Evidence thus far shows that this form of cancer acts more like a solid tumor and treatment options should be explored on a case-specific basis.
Very few occurrences of bilateral disease have been described in literature. More research is required to determine the need for contralateral capsulectomy and the risk of recurrence with reimplantation. It is important to closely surveil patients who have undergone unilateral capsulectomy and implant removal for development of contralateral disease in the interim until definitive data is collected.
Conclusions
A collaborative effort between a multispecialty team is an important aspect of BIA-ALCL diagnosis and treatment. Early recognition of this rare cancer, complete capsulectomy and implant removal, and in appropriate instances, chemotherapy can ensure a positive outcome for future patients with BIA-ALCL. Involvement of plastic surgery after the initial discovery of a breast mass in association with a breast implant capsule is important to establish long term goals of care and a comprehensive plan for treatment.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/abs.2019.01.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Laurent C, Delas A, Gaulard P, et al. Breast implant-associated anaplastic large cell lymphoma: two distinct clinicopathological variants with different outcomes. Ann Oncol 2016;27:306-14. [Crossref] [PubMed]
- Ravi-Kumar S, Sanaei O, Vasef M, et al. Anaplastic large cell lymphoma associated with breast implants. World J Plast Surg 2012;1:30-5. [PubMed]
- Clemens MW, Medeiros LJ, Butler CE, et al. Complete Surgical Excision Is Essential for the Management of Patients With Breast Implant-Associated Anaplastic Large-Cell Lymphoma. J Clin Oncol 2016;34:160-8. [Crossref] [PubMed]
- Johnson L, O'Donoghue JM, McLean N, et al. Breast implant associated anaplastic large cell lymphoma: The UK experience. Recommendations on its management and implications for informed consent. Eur J Surg Oncol 2017;43:1393-401. [Crossref] [PubMed]
Cite this article as: Fine KE, Wi M, Bennett DC. Breast implant-associated anaplastic large cell lymphoma (BIA-ALCL): a collaborative effort for diagnosis and treatment. Ann Breast Surg 2019;3:1.

