
Prone liver phase MRA demonstrates improved intramuscular vascular detail compared to CTA in preoperative perforator mapping for free autologous abdominally-based breast reconstruction
Introduction
Free autologous abdominally-based breast reconstruction is a reliable technique for producing a natural result after mastectomy by restoring soft tissue volume and contour while reconstituting the skin envelope of the breast. Compared to the free transverse rectus abdominis myocutaneous (TRAM) flap, the deep inferior epigastric artery perforator (DIEP) flap or muscle-sparing flaps are advantageous because of decreased abdominal morbidity. However, there is an increased risk of fat necrosis and partial flap loss (1-4). As muscle-sparing and perforator flap techniques have evolved, radiographic imaging has been used to pre-operatively design flaps based on a patient’s unique anatomy with the goal of decreasing operative time and minimizing complications (2,5).
CTA, MRA, and Doppler sonography have been used for pre-operative planning of free flap harvest for breast reconstruction and have been shown to reliably predict abdominal wall anatomy (5-11). Preoperative imaging is commonly used to facilitate planning and execution of abdominal perforator flap harvest by demonstrating cutaneous perforator location and deep inferior epigastric or superficial inferior epigastric artery dominance and branching pattern. Pre-operative imaging facilitates flap dissection and allows the surgeon to provide personalized informed consent by understanding the suitability of the patient’s vascular anatomy before surgery (8,12-14). CTA and MRA are most commonly employed for pre-operative planning due to their reliability and availability, whereas Doppler ultrasonography performance can suffer from technician variability (6,7).
Prone liver phase MRA (PLP-MRA) is a 3D volumetric fat-suppressed gradient echo technique that has been shown to have superior muscle to vascular detail compared to CTA (15-17). The liver phase MRA protocol allows combined arterial and venous detail (Figure 1), with markedly enhanced contrast between the vessels and surrounding muscles compared to CTA (15). Additionally, it does not expose the patient to ionizing radiation. The simultaneous enhancement of perforating arteries and their paired veins with MRA enables better selection of optimal perforators (17). Both 1.5-T and 3.0-T MRI scanners can be used for this purpose, but 1.5-T is preferred due to less suppression of the surrounding muscle, leading to enhancement of the relationship between perforators and muscle (16).
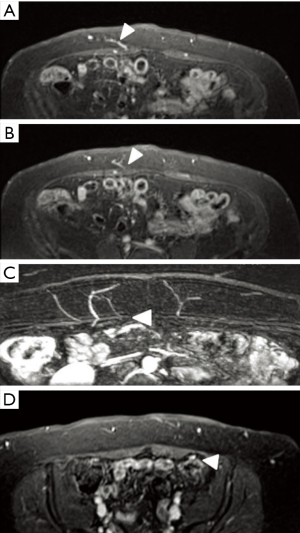
Pre-operative imaging to plan flap-based reconstruction is often scheduled after consultation for breast reconstruction, in addition to other imaging studies such as breast MRI or PET CT, which are used for staging (18-20). PLP-MRA can be performed concomitantly with breast MRI. It is available in many tertiary care centers, allowing for preoperative planning for flap selection during the breast cancer workup. Furthermore, it has the potential to improve convenience in appropriately selected patients by minimizing the number of imaging modalities employed in their treatment.
With CTA having widespread use in operative planning of free autologous abdominally-based breast reconstruction, this modality was selected as the comparison for PLP-MRA to study its performance in evaluating abdominal wall vascular anatomy (12).
Methods
Imaging modality selection
Informed consent documentation was obtained from each patient prior to participation based on a protocol approved by the Institutional Review Board at Virginia Commonwealth University (IRB #13438). Twenty-four consecutive patients electing to proceed with free autologous abdominally-based breast reconstruction were randomized to receive PLP-MRA or CTA using a random number generator with the code protected by the radiographic study interpreter (Randomness and Integrity Services, Ltd. Dublin, Ireland). Patients were randomized to either PLP-MRA or CTA (as opposed to obtaining both studies) due to the need to obtain insurance authorization for the imaging. Any female patient with a desire to undergo breast reconstruction was eligible for inclusion regardless of the need for delayed or immediate reconstruction. Patients with an indwelling vascular access device or breast tissue expander were excluded from the study due to the inability to randomize these patients to PLP-MRA. Age, BMI, smoking status, prior medical conditions, and prior abdominal surgery were compared to ensure the fidelity of the randomization. Medical conditions recorded in this study include diabetes, hypertension, and history of coronary artery disease. All patients in this study had a minimum of one-year follow-up.
Radiographic outcome variables
CTA and PLP-MRA imaging were used to predict whether a DIEP or muscle-sparing free TRAM flap would be performed according to the senior author’s interpretation of the studies. Briefly, dominant perforators greater than 1.5 mm in diameter were identified. If they were aligned vertically where rectus muscle sacrifice for dissection was not required, a DIEP flap was predicted. If there were no decidedly dominant perforators, or if perforator dominance appeared to be shared equally between medial and lateral systems, a muscle-sparing-2-TRAM (MS2-TRAM) free flap was predicted. The senior author devised this classification system to be utilized preoperatively by the co-authors, but he was blinded to both the scans (CTA and PLP-MRA) and their interpretation until completion of the operation.
Location of the cutaneous perforators relative to the umbilicus on CTA or PLP-MRA was correlated with intra-operative findings for all patients (Figure 2). The intramuscular pedicle length on pre-operative imaging was measured to determine the amount of muscular dissection that would be required for perforator flap dissection. For patients that underwent DIEP flap dissection, this distance was compared to the intraoperatively-measured intramuscular pedicle distance (Figure 3). The distance of the pedicle from the semilunar line at the most proximal and distal perforator was measured pre-operatively to help determine where the rectus sheath should be divided to initiate MS-2-TRAM free flap harvest. For patients undergoing MS-2-TRAM flap harvest, this distance was compared to intra-operative measurements at the most proximal and distal perforators, and both distances were averaged for imaging-derived and surgical-derived values separately (Figure 4). All radiographic endpoints were measured in a blinded manner with one study author determining the perforator location in each imaging study and the senior author recording perforator location intra-operatively. Table 1 describes radiographic outcome measures and the potential impact on free flap design.
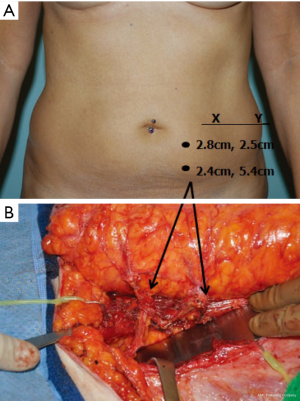
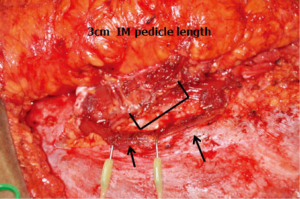
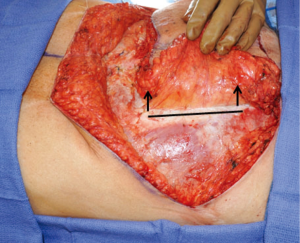
Table 1
| Measurement | Application |
|---|---|
| Perforator location relative to the umbilicus | Perforators >1.5 mm in diameter are identified. Their location relative to the umbilicus provides information regarding the site of meticulous perforator dissection. Perforator identification helps to distinguish medial from lateral row dominance and DIEP flap versus MS-2-TRAM free flap candidates |
| Intramuscular pedicle length | Abdominal anatomy with short intra-muscular pedicle length requires less intra-muscular dissection and may be more appropriate for DIEP flap harvest compared to vessels requiring a significant length of intra-muscular dissection |
| Distance of pedicle from semilunar line | Position of lateral fascial incision for MS-2-TRAM flap harvest and knowledge of locations requiring meticulous dissection for muscle division based on pedicle location |
Statistics
Age and BMI between the two groups were compared using t-test, and medical/surgical history was compared between the two groups using Fisher’s exact test to confirm randomization across all patient attributes.
Paired radiographic and intra-operative measurements of perforator location relative to the umbilicus, intramuscular pedicle length, and pedicle position relative to the semilunar line were compared by paired Wilcoxon rank sum test.
An effect size of one-centimeter difference in a single direction between radiographic and intra-operative assessment was selected for power calculation based on a surgeon focus group consensus of an important clinical difference. Power calculation for perforator location was determined with standard deviations taken from the literature using CTA and MRA (5,9,13-15,21,22). For distance from the semilunar line and intra-muscular pedicle length, thirty CTAs of the abdomen not included in this study were reviewed to provide a radiographic standard deviation for these variables to calculate paired sample size needed. Thirty-four perforators, 24 pedicle lengths, and 10 pedicle distances from the semilunar line would be required to provide 80% power to detect a 1-cm difference in radiographic versus intra-operative assessments with a P value of 0.05. Randomization was terminated when the appropriate number of radiographic outcomes was accumulated to power the study (21,23).
Results
A total of 24 patients were randomized to preoperative imaging by CTA (12) or PLP-MRA (12) with a total of 30 flaps (6 patients were bilateral). In the PLP-MRA group, 5 patients received DIEP flaps and 9 patients received MS2-TRAM flaps. In the CTA group, 7 patients received DIEP flaps and 9 patients received MS2-TRAM flaps (Table 2).
Table 2
| Variables | Randomized imaging type | |||
|---|---|---|---|---|
| PLP-MRA (n=12) | CTA (n=12) | |||
| Branching pattern | ||||
| I | 3 | 2 | 5 | 2 |
| II | 1 | 7 | 2 | 7 |
| III | 1 | 0 | 0 | 0 |
| Flap type | 5 (DIEP) | 9 (MS-2-TRAM) | 7 (DIEP) | 9 (MS-2-TRAM) |
DIEP, deep inferior epigastric artery perforator; MS2-TRAM, muscle-sparing-2-transverse rectus abdominis myocutaneous.
The mean ages were 47 [39–51] in the PLP-MRA group and 49 [40–61] in the CTA group. The mean BMI was 31 [23–45] in the PLP-MRA group and 29 [19–39] in the CTA group. There were 3 patients with medical comorbidities in the PLP-MRA group (2 with diabetes and 1 with hypertension) versus 4 patients in the CTA group (2 with hypertension, 1 with diabetes, and 1 with coronary artery disease and hypertension). Four patients in the PLP-MRA group had a history of abdominal surgery versus 5 patients in the CTA group. There were 3 smokers in the MRA group and 4 smokers in the CTA group (Table 3).
Table 3
| Imaging type | MRA | CTA | P |
|---|---|---|---|
| Demographics | |||
| Age | 47 [39–51] | 49 [40–61] | 0.82 |
| BMI | 31 [23–45] | 29 [19–39] | 0.60 |
| Medical history | |||
| Medical comorbidities | 3 | 4 | 0.24 |
| Prior abdominal surgery | 4 | 5 | 0.40 |
| Smoking | 3 | 4 | 0.24 |
Radiographic and intra-operative perforator locations differed in the PLP-MRA group by a mean of 0.78 cm (0–1.3 cm) on the x-axis (P=0.62) and by 0.80 cm (0–2.4 cm) on the y-axis (P=0.08) and in the CTA group by 0.69 cm (0–1.2 cm) on the x-axis (P=0.30) and by 0.82 cm (0–2.2 cm) on the y-axis (P=0.20). Both CTA and PLP-MRA accurately predicted perforator location with no significant differences between radiographic or intra-operative assessment. Forty-seven perforators greater than 1.5 mm in diameter were identified in the PLP-MRA group, and fifty were identified in the CTA group (Table 4).
Table 4
| Variables | Mean difference (range), cm | P |
|---|---|---|
| PLP-MRA (n=47) | ||
| x-coordinates | 0.78 (0–1.3) | 0.62 |
| y-coordinates | 0.80 (0–2.4) | 0.08 |
| CTA (n=50) | ||
| x-coordinates | 0.69 (0–1.2) | 0.30 |
| y-coordinates | 0.82 (0–2.2) | 0.20 |
The difference between the mean radiographic and intra-operative intramuscular pedicle length was 0.56 cm (0.3–1.1 cm) in the PLP-MRA group (P=0.16) and 1.73 cm (0.5–2.6 cm) in the CTA group (P=0.045). While PLP-MRA measurements of intramuscular pedicle length agreed with intra-operative findings, CTA was found to be significantly different than intra-operative assessments (Table 5).
Table 5
| Variables | Mean (standard deviation), cm | P |
|---|---|---|
| PLP-MRA (n=14) | 0.16 | |
| PLP-MRA IM pedicle length | 6.55 (2.42) | |
| Intra-operative IM pedicle length | 6.29 (1.88) | |
| Imaging vs. anatomic difference | 0.56 (0.32) | |
| CTA (n=16) | 0.045 | |
| CTA IM pedicle length | 6.05 (1.65) | |
| Intra-operative IM pedicle length | 5.04 (1.25) | |
| Imaging vs. anatomic difference | 1.73 (0.55) |
n, number of hemi-abdomens with perforator flap dissection.
The difference between the mean radiographic and intra-operative distance of the pedicle from the semilunar line was 0.41 cm (0–1 cm) in the PLP-MRA group (P=0.767) and 0.62 cm (0–1.5 cm) in the CTA group (P=0.790) (Table 6). Both imaging modalities accurately predicted pedicle distance from the semilunar line.
Table 6
| Variables | Mean (standard deviation), cm | P |
| MRA (n=12) | ||
| PLP-MRA distal position | 3.53 (1.28) | 0.54 |
| Surgery distal position | 3.46 (0.84) | |
| PLP-MRA proximal position | 2.58 (0.70) | 0.58 |
| Surgery proximal position | 2.58 (1.02) | |
| CTA (n=12) | ||
| CTA distal position | 3.29 (1.03) | 0.45 |
| Surgery distal position | 3.13 (0.48) | |
| CTA proximal position | 2.21 (0.99) | 0.40 |
| Surgery proximal position | 2.46 (0.62) |
Discussion
In this study we demonstrated the utility of PLP-MRA to identify radiographic measures that are useful in planning and performing free autologous abdominally-based breast reconstruction. This MRA protocol can be performed concomitantly with staging breast MRI without the need for ionizing radiation, and it was shown to accurately depict perforator location relative to the umbilicus and from the semilunar line. It also provided additional accuracy when localizing the pedicle’s intramuscular course when compared to CTA.
Standard MRA and CTA have been directly compared in a study by Cina and colleagues, which showed that MRA is a reliable method for mapping DIEPs (24). Greenspun and colleagues used an MRI protocol that studied patients in the prone position using a 1.5-T system with gadolinium based contrast, and based on comparison of their results with similar studies of CTA, concluded that the accuracy of MRA was comparable to CTA for determining perforator location (13). They also noted that prone positioning allows for superior perforator imaging with MRA due to reduced motion artifact through reduced respiratory motion.
Another benefit of preoperative imaging is surgical efficiency. Preoperative knowledge of DIEP anatomy has been shown to reduce operative time for DIEP flap harvest (21,22,25-27). The results of our study demonstrate that PLP-MRA provided a significantly more accurate prediction of the intramuscular pedicle length than did CTA. Having this knowledge preoperatively enables the surgeon to predict potential challenges in perforator dissection. Used together with information about the size and number of perforators, a determination about feasibility of DIEP flap harvest can be made with these measurements.
If the pre-operative decision is made for a muscle-sparing design, pedicle distance from the semilunar line can provide guidance on placement of the lateral rectus fascia and muscle incision for flap harvest, and precise locations of perforators will allow the surgeon to know where meticulous dissection is most important (Table 6).
Magnetic resonance imaging has become a useful adjunct in evaluation for select cases of breast cancer, including the ability to identify and characterize lesions in a dense breast that cannot be thoroughly evaluated by mammography, localization of lesions seen on only one mammographic projection, improved characterization of benign and malignant masses, better evaluation of tumor extent, evaluation of the contralateral breast, evaluation of axillary adenopathy, and more accurate surveillance in the post-lumpectomy breast (28,29). PLP-MRA is widely available and this imaging technique can be added to the breast MRI imaging protocol to enable that all imaging is carried out in a single session. This has the potential to streamline the care of the breast cancer patient, thereby improving their overall care experience. The concept of seamless delivery of healthcare services has been identified as an effective method to promote optimum patient outcomes and improve patient satisfaction in cancer care (30).
Additionally, there is increasing attention on behalf of physicians and patients on radiation exposure and the increased risk of malignancy associated with the widespread use of CT imaging. This is of particular concern for female patients, who are reported to have a higher risk of cancer development compared to men as a result of CT-associated radiation exposure (31). Moreover, the risk of acute allergic reactions and renal damage due to intravascular contrast administration are lower with gadolinium-based studies such as MRA as compared with iodinated contrast agents used in CTA (24).
As MRI is indicated as part of the imaging workup of a select group of high-risk breast cancer patients, the use of PLP-MRA for both breast imaging and planning of breast reconstruction in these patients would obviate the need for CTA thereby eliminating the risk of future cancer development secondary to radiation exposure, decreasing the chance of allergic reaction and renal damage due to IV contrast, and eliminating treatment delays associated with multiple imaging procedures. Patients who are candidates for free autologous abdominally-based breast reconstruction that do not require MRI as part of their breast cancer workup would also benefit from PLP-MRA solely for preoperative planning, as they would avoid exposure to ionizing radiation from CT and its associated risks. The images from a PLP-MRA are similar to those generated by CT, and the vascular anatomy of the abdominal wall can be easily interpreted by non-radiologist physicians with experience interpreting CT images, making this modality a convenient alternative to CT for the plastic surgeon planning a free tissue transfer.
Conclusions
This study demonstrates that PLP-MRA offers superior accuracy in predicting intramuscular pedicle length compared to CTA, while maintaining accuracy in determining perforator location and pedicle position to assist with flap design. This MRA protocol can be performed in tandem with breast staging MRI, thereby subjecting patients to fewer imaging studies, avoiding treatment delays, decreasing operating time, and preventing exposure to ionizing radiation.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/abs.2018.06.02). Podium presentation at the American Society for Reconstructive Microsurgery, Las Vegas, NV, USA, January 2012; Poster Presentation at the 57th Annual Plastic Surgery Research Council, Ann Arbor, MI, USA, May 2012. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board at Virginia Commonwealth University (No. #13438) and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Man LX, Selber JC, Serletti JM. Abdominal wall following free TRAM or DIEP flap reconstruction: a meta-analysis and critical review. Plast Reconstr Surg 2009;124:752-64. [Crossref] [PubMed]
- Rozen WM, Stella DL, Bowden J, et al. Advances in the pre-operative planning of deep inferior epigastric artery perforator flaps: magnetic resonance angiography. Microsurgery 2009;29:119-23. [Crossref] [PubMed]
- Selber JC, Nelson J, Fosnot J, et al. A prospective study comparing the functional impact of SIEA, DIEP, and muscle-sparing free TRAM flaps on the abdominal wall: part I. unilateral reconstruction. Plast Reconstr Surg 2010;126:1142-53. [Crossref] [PubMed]
- Vyas RM, Dickinson BP, Fastekjian JH, et al. Risk factors for abdominal donor-site morbidity in free flap breast reconstruction. Plast Reconstr Surg 2008;121:1519-26. [Crossref] [PubMed]
- Hijjawi JB, Blondeel PN. Advancing deep inferior epigastric artery perforator flap breast reconstruction through multidetector row computed tomography: an evolution in preoperative imaging. J Reconstr Microsurg 2010;26:11-20. [Crossref] [PubMed]
- Giunta RE, Geisweid A, Feller AM. The value of preoperative Doppler sonography for planning free perforator flaps. Plast Reconstr Surg 2000;105:2381-6. [Crossref] [PubMed]
- Hallock GG. Acoustic Doppler sonography, color duplex ultrasound, and laser Doppler flowmetry as tools for successful autologous breast reconstruction. Clin Plast Surg 2011;38:203-11. [Crossref] [PubMed]
- Mathes DW, Neligan PC. Preoperative imaging techniques for perforator selection in abdomen-based microsurgical breast reconstruction. Clin Plast Surg 2010;37:581-91. xi. [Crossref] [PubMed]
- Neil-Dwyer JG, Ludman CN, Schaverien M, et al. Magnetic resonance angiography in preoperative planning of deep inferior epigastric artery perforator flaps. J Plast Reconstr Aesthet Surg 2009;62:1661-5. [Crossref] [PubMed]
- Schaverien MV, Ludman CN, Neil-Dwyer J, et al. Contrast-enhanced magnetic resonance angiography for preoperative imaging in DIEP flap breast reconstruction. Plast Reconstr Surg 2011;128:56-62. [Crossref] [PubMed]
- Smit JM, Klein S, Werker PM. An overview of methods for vascular mapping in the planning of free flaps. J Plast Reconstr Aesthet Surg 2010;63:e674-82. [Crossref] [PubMed]
- Chernyak V, Rozenblit AM, Greenspun DT, et al. Breast reconstruction with deep inferior epigastric artery perforator flap: 3.0-T gadolinium-enhanced MR imaging for preoperative localization of abdominal wall perforators. Radiology 2009;250:417-24. [Crossref] [PubMed]
- Greenspun D, Vasile J, Levine JL, et al. Anatomic imaging of abdominal perforator flaps without ionizing radiation: seeing is believing with magnetic resonance imaging angiography. J Reconstr Microsurg 2010;26:37-44. [Crossref] [PubMed]
- Smit JM, Dimopoulou A, Liss AG, et al. Preoperative CT angiography reduces surgery time in perforator flap reconstruction. J Plast Reconstr Aesthet Surg 2009;62:1112-7. [Crossref] [PubMed]
- Schaverien MV, Ludman CN, Neil-Dwyer J, et al. Contrast-enhanced magnetic resonance angiography for preoperative imaging of deep inferior epigastric artery perforator flaps: advantages and disadvantages compared with computed tomography angiography: a United Kingdom perspective. Ann Plast Surg 2011;67:671-4. [Crossref] [PubMed]
- Vasile JV, Newman T, Rusch DG, et al. Anatomic imaging of gluteal perforator flaps without ionizing radiation: seeing is believing with magnetic resonance angiography. J Reconstr Microsurg 2010;26:45-57. [Crossref] [PubMed]
- Vasile JV, Newman TM, Prince MR, et al. Contrast-enhanced magnetic resonance angiography. Clin Plast Surg 2011;38:263-75. [Crossref] [PubMed]
- Teller P, Jefford VJ, Gabram SG, et al. The utility of breast MRI in the management of breast cancer. Breast J 2010;16:394-403. [PubMed]
- Thibault F, Nos C, Meunier M, et al. MRI for surgical planning in patients with breast cancer who undergo preoperative chemotherapy. AJR Am J Roentgenol 2004;183:1159-68. [Crossref] [PubMed]
- Yeh ED. Breast magnetic resonance imaging: current clinical indications. Magn Reson Imaging Clin N Am 2010;18:155-69. vii. [Crossref] [PubMed]
- Alonso-Burgos A, Garcia-Tutor E, Bastarrika G, et al. Preoperative planning of DIEP and SGAP flaps: preliminary experience with magnetic resonance angiography using 3-tesla equipment and blood-pool contrast medium. J Plast Reconstr Aesthet Surg 2010;63:298-304. [Crossref] [PubMed]
- Kuekrek H, Muller D, Paepke S, et al. Preoperative CT angiography for planning free perforator flaps in breast reconstruction. Handchir Mikrochir Plast Chir 2011;43:88-94. [Crossref] [PubMed]
- Delucchi KL. Sample size estimation in research with dependent measures and dichotomous outcomes. Am J Public Health 2004;94:372-7. [Crossref] [PubMed]
- Cina A, Barone-Adesi L, Rinaldi P, et al. Planning deep inferior epigastric perforator flaps for breast reconstruction: a comparison between multidetector computed tomography and magnetic resonance angiography. Eur Radiol 2013;23:2333-43. [Crossref] [PubMed]
- Cina A, Salgarello M, Barone-Adesi L, et al. Planning breast reconstruction with deep inferior epigastric artery perforating vessels: multidetector CT angiography versus color Doppler US. Radiology 2010;255:979-87. [Crossref] [PubMed]
- Masia J, Clavero JA, Larranaga JR, et al. Multidetector-row computed tomography in the planning of abdominal perforator flaps. J Plast Reconstr Aesthet Surg 2006;59:594-9. [Crossref] [PubMed]
- Rozen WM, Garcia-Tutor E, Alonso-Burgos A, et al. Planning and optimising DIEP flaps with virtual surgery: the Navarra experience. J Plast Reconstr Aesthet Surg 2010;63:289-97. [Crossref] [PubMed]
- Bassett LW SM. The breast: comprehensive management of benign and malignant disorders. 3rd ed. St Louis, MO: Elsevier, 2004.
- Lyman GH, Baker J, Geradts J, et al. Multidisciplinary care of patients with early-stage breast cancer. Surg Oncol Clin N Am 2013;22:299-317. [Crossref] [PubMed]
- Drury VB, Inma C. Exploring patient experiences of cancer services in regional Australia. Cancer Nurs 2010;33:E25-31. [Crossref] [PubMed]
- Smith-Bindman R, Lipson J, Marcus R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med 2009;169:2078-86. [Crossref] [PubMed]
Cite this article as: Olenczak JB, Martinovic M, Martin JP, Campbell CA. Prone liver phase MRA demonstrates improved intramuscular vascular detail compared to CTA in preoperative perforator mapping for free autologous abdominally-based breast reconstruction. Ann Breast Surg 2018;2:12.

