The impact of partial breast reconstruction with lateral chest wall perforator flaps on post-operative cancer surveillance
Introduction
Oncoplastic breast-conserving surgery refers to breast conserving surgery (BCS) offered to women with relatively large or poorly located cancers and involves specialized plastic surgical techniques in combination with oncological surgery (1,2). With the development and popularization of partial breast reconstruction (PBR), in particular volume replacement (3-5) with chest wall perforator flaps (CWPFs), it has extended the indications for BCS for women diagnosed with breast cancer with improved aesthetic and psychological outcomes.
CWPF is a relatively new technique, gradually gaining interest and acceptance. The history of use of lateral chest wall flaps for breast reconstruction dates back to 1986 where Holmstrom et al. (6) described the use of a lateral thoracodorsal flap to assist in implant reconstruction after mastectomy for breast cancer. Subsequently, it was described in detail by Hamdi et al. in 2004 (7-9) as a pedicled perforated flap based on various named perforators: the lateral intercostal artery perforators (LICAPs), the branch of the lateral thoracic artery (LTAP) and the thoracodorsal artery perforator (TDAP).
Since then, CWPFs have been increasing in popularity as an option for BCS in women with small to moderate non-ptotic breasts with laterally placed tumours (10). However, many people fear that PBR with CWPF may alter the breast architecture in some way and hence affect the patterns of local recurrence and make postoperative cancer surveillance more difficult. The importance of accurate surveillance (11-14) cannot be over-emphasised as it is the hallmark of oncoplastic BCS where safety of procedure in terms of oncologic clearance and surveillance carries significant importance.
As this is a less widely used technique, there is paucity of literature (15,16) reporting the effects of these procedures on the evaluation of subsequent surveillance mammograms.
The aim of this paper is to characterize the mammographic findings of post BCS breast after volume replacement and evaluate the outcomes and impact on surveillance mammograms.
Methods
This is a retrospective analysis of a prospectively maintained database of all patients who underwent PBR with CWPF as part of BCS by a single surgeon in a tertiary referral centre. The CWPF are fasciocutaneous pedicled flaps based on either the LICAP, branch of LTAP or TDAP. It is designed on the lateral chest wall by pinching the redundant roll of fat with variable extension around the back, depending on the tissue needed to fill the defect. The flap is orientated parallel to the skin tension lines with the tip curving up posteriorly parallel to the underlying ribs and following angiosome description (17).
Surveillance mammograms are those performed in asymptomatic patients following initial treatment of a primary breast cancer. This is usually performed at one-year after surgery and annually thereafter for at least five years as per NHS guidance (18) on surveillance for breast cancer. Symptomatic patients have access to symptom review clinic at all times and are referred for diagnostic imaging when necessary.
Mammograms done after surgery were reviewed and reported by consultant breast radiologists at the point of imaging. The need for recall for further imaging and/or biopsy were performed as indicated. All available imaging was retrieved for this study. The first post-operative surveillance mammogram was analysed for characteristic qualitative features. Outcomes of surveillance, whether normal or recalled for additional imaging/biopsy, were analysed. An interval cancer (19) is defined as a cancer diagnosed within the breast after a negative surveillance mammogram. The incidence and outcomes of diagnostic imaging for symptomatic patients were also evaluated.
The study was carried out as a part of routine clinical care with approval (approval number 4371) from the ethics committee of Oxford University Hospitals NHS trusts to audit the outcomes. The hospital ethical and clinical guidelines were adhered to.
The data were statistically described in terms of mean, median and range, or frequencies (number of cases) and percentages when appropriate.
Results
Sixty-four women diagnosed with breast cancer underwent volume replacement BCS over the study period (Aug 2011–Apr 2016). All were females and the median age at diagnosis was 50 years (range: 34–69 years). Most presented with symptoms whilst 29.7% (19/64) were screen-detected. Most patients underwent volume replacement BCS in a single stage procedure whereby the wide local excision and CWPF was performed in a single operation. A small number (6/64) underwent a two-stage procedure (10,20) whereby the wide local excision was performed in the first operation, the cavity was filled with saline and the CWPF was performed in the second operation. And 14% (9/64) of the patients had the surgery after neo-adjuvant chemotherapy.
The median follow-up was 2 years (range: 1–5 years). Four patients (6.3%) required completion mastectomy for incomplete cancer resection, which is an acceptable rate when compared with standard BCS.
A total of 58 female patients who had at least one surveillance mammograms post-operatively were included in the analysis. Six patients were excluded as 4 subsequently underwent completion mastectomy, 1 refused mammographic surveillance and 1 had yet to have their first post-operative surveillance mammogram.
Characteristic mammographic features at one-year post surgery
As CWPF uses subcutaneous adipose tissue and dermis from the lateral chest wall to replace the volume of the resected breast, the flap is only visible in 13.8% of the mammograms performed at one-year post surgery. Most (77.6%) show intermediate parenchymal density. Of the 58 mammograms reviewed, 5 showed calcifications, mostly benign except for 1 which showed fine calcifications. Fat necrosis was seen in 2 of the 58 mammograms and up to 20% showed post-radiation changes such as fat necrosis or skin thickening. The typical features of CWPF on the first post-operative mammograms were reviewed in Table 1 and Figure 1 shows typical post-operative mammograms.
Table 1
| Qualitative features | n | Percentage (%) |
|---|---|---|
| Parenchymal density | ||
| Dense/moderately dense | 6 | 10.3 |
| Intermediate | 45 | 77.6 |
| Fatty | 7 | 12.1 |
| Calcifications | 5 | 8.6 |
| Mass | 0 | 0 |
| Fat necrosis | 2 | 3.4 |
| Skin thickening | 12 | 20.7 |
| Breast edema | 7 | 12.1 |
| Flap seen distinctly | 8 | 13.8 |

Outcomes of surveillance mammography
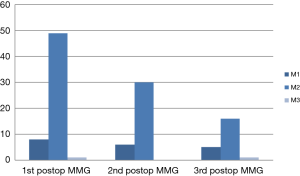
In total, 134 mammograms were reviewed in this study. The UK 5-point breast imaging classification for the mammograms done in the first 3 post-operative years are presented in Figure 2. All were labelled as M1 (normal) and M2 (benign) while only 2 were M3 (indeterminate/probably benign).
Three mammograms (2.2%) were recalled for further imaging. All had additional ultrasound evaluation and 1 had spot compression and/or magnification view mammography. Magnetic resonance imaging was not required in any patient. All 3 patients proceeded to have core biopsy of the mammographic abnormality. The biopsy results were 2 benign lesions (B2) including fat necrosis, fibroadenoma while one showed atypia (B3). The patient with B3 on biopsy subsequently underwent wire guided excision biopsy which confirmed benign calcifications with atypia.
Post-radiation changes including skin thickening & breast oedema were seen in 17.2% (23/134) mammograms, mainly in first couple of years, which resolved by the third surveillance mammogram. Mammographic evidence of fat necrosis was seen in 3.7% (5/134) and 1 required biopsy. There were no cases of interval cancer or recurrence.
Diagnostic imaging after CWPF
Diagnostic imaging was performed for evaluation of patient-reported symptoms and/or clinician-detected signs within the post-operative breast. Over the duration of follow-up, 4 patients (6.9%) presented with symptoms which led to additional, unplanned imaging. Three of the patients presented in the clinic with firmness or nodularity and 1 presented with axillary pain. All the patients had ultrasound of the area of concern. All the ultrasound was normal/benign and showed post-operative changes while 1 showed fat necrosis. The patient who presented with nodularity and had ultrasound showing fat necrosis eventually underwent a core biopsy for the area of concern and the biopsy confirmed fat necrosis and fibrosis.
Discussion
Volume replacement techniques allow women, who would otherwise have been rendered a mastectomy, to have the option of BCS. With increasing popularity of reconstructive techniques for BCS in women with breast cancer, the importance of adequate postoperative surveillance cannot be emphasized enough.
However, as volume replacement or PBR using CWPF is a relatively new technique, the oncological safety aspect in terms of impact on surveillance mammograms in detecting recurrences/new cancers need to be evaluated. The mammograms of such patients after CWPF are unique in the sense that the flap may or may not be visible due to the absence of muscle density which is commonly seen in patients who had undergone LD flaps (15). A similar study by Nottingham group (16) looking at mammograms after volume replacement showed that the evidence of an autologous flap was absent in 31% of those who had CWPF. Furthermore, there was less architectural distortion and when present, it lacks the spiculate appearance noted after the usual BCS (14,21). The incidence of fat necrosis among the surveillance mammogram in our study population is 3.7% and it compares very favourably with the 20–31.9% incidence of fat necrosis seen after standard BCS with wide local excision (22,23).
Surveillance mammographic follow-up after PBR with CWPF in women undergoing BCS for breast cancer is accurate with low recall & biopsy rates. In the 134 surveillance mammograms we reviewed, the recall rate was only 2.2%. This is comparable to the recall rate of post-treatment surveillance mammogram in our centre which is 3.7%. During the same period, a biopsy recommendation resulting in a benign histology results occurred in 2 of 134 mammograms, giving a false positive rate of 1.5%. This is comparable to the study by Ashkanani et al. (24) which reported a false positive rate of 2.3% and also the Nottingham paper which quoted 0.67%.
We also looked at the impact of volume replacement BCS on clinical resources in terms of additional imaging and need for biopsy for patients who presented symptomatically. During the follow-up period, 4 patients required additional imaging, mainly in the form of ultrasound. Ultrasonographic evaluation alone was adequate to exclude any suspicious lesions in most of the patients and only 1 patient required a biopsy.
Our study is limited by the relatively small sample size and short duration of follow-up. However, it provides reassurance to the units wishing to adopt the new technique and expand their repertoire in oncoplastic breast surgery techniques. There is continuing need to evaluate the oncoplastic techniques with inbuilt quantitative and qualitative audits across various units and hopefully as collective experience expands, there will be more information available on the outcomes and impact of volume replacement BCS.
Acknowledgments
We would like to acknowledge Dr Victoria Chan for her help in providing some of the statistics involved.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/abs.2018.04.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was carried out as a part of routine clinical care with approval (approval number 4371) from the ethics committee of Oxford University Hospitals NHS trusts to audit the outcomes. The hospital ethical and clinical guidelines were adhered to. Informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Clough KB, Lewis JS, Couturaud B, et al. Oncoplastic techniques allow extensive resections for breast-conserving therapy of breast carcinomas. Ann Surg 2003;237:26-34. [Crossref] [PubMed]
- Regaño S, Hernanz F, Ortega E, et al. Oncoplastic techniques extend breast-conserving surgery to patients with neoadjuvant chemotherapy response unfit for conventional techniques. World J Surg 2009;33:2082-6. [Crossref] [PubMed]
- Losken A, Hamdi M. Partial breast reconstruction: current perspectives. Plast Reconstr Surg 2009;124:722-36. [Crossref] [PubMed]
- McCulley SJ, Schaverien MV, Tan VK, et al. Lateral thoracic artery perforator (LTAP) flap in partial breast reconstruction. J Plast Reconstr Aesthet Surg 2015;68:686-91. [Crossref] [PubMed]
- Munhoz AM, Montag E, Arruda E, et al. Immediate conservative breast surgery reconstruction with perforator flaps: new challenges in the era of partial mastectomy reconstruction? Breast 2011;20:233-40. [Crossref] [PubMed]
- Holmström H, Lossing C. The lateral thoracodorsal flap in breast reconstruction. Plast Reconstr Surg 1986;77:933-43. [Crossref] [PubMed]
- Hamdi M, Van Landuyt K, de Frene B, et al. The versatility of the inter-costal artery perforator (ICAP) flaps. J Plast Reconstr Aesthet Surg 2006;59:644-52. [Crossref] [PubMed]
- Hamdi M, Van Landuyt K, Hijjawi JB, et al. Surgical technique in pedicled thoracodorsal artery perforator flaps: a clinical experience with 99 patients. Plast Reconstr Surg 2008;121:1632-41. [Crossref] [PubMed]
- Hamdi M, Van Landuyt K, Monstrey S, et al. Pedicled perforator flaps in breast reconstruction: a new concept. Br J Plast Surg 2004;57:531-9. [Crossref] [PubMed]
- Roy P. One-stage vs. two-stage approach for partial breast reconstruction with lateral chest wall perforator flaps. Cancer Treat Res Commun 2016;9:56-61. [Crossref]
- Ciatto S, Cataliotti L, Pacini P, et al. Cancer reoccurrence in the conserved breast - diagnostic features in a consecutive series of 102 cases. Int J Oncol 1993;3:337-9. [PubMed]
- Houssami N, Ciatto S. Mammographic surveillance in women with a personal history of breast cancer: how accurate? How effective? Breast 2010;19:439-45. [Crossref] [PubMed]
- Dershaw DD, McCormick B, Osborne MP. Detection of local recurrence after conservative therapy for breast carcinoma. Cancer 1992;70:493-6. [Crossref] [PubMed]
- Dershaw DD. Mammography in patients with breast cancer treated by breast conservation (lumpectomy with or without radiation). AJR Am J Roentgenol 1995;164:309-16. [Crossref] [PubMed]
- Monticciolo DL, Ross D, Bostwick J, et al. Autologous breast reconstruction with endoscopic latissimus dorsi musculosubcutaneous flaps in patients choosing breast-conserving therapy: mammographic appearance. AJR Am J Roentgenol 1996;167:385-9. [Crossref] [PubMed]
- Tan VK, Cornford EJ, McCulley SJ, et al. Qualitative mammographic findings and outcomes of surveillance mammography after partial breast reconstruction with an autologous flap. J Surg Oncol 2015;111:377-81. [Crossref] [PubMed]
- Taylor GI. The angiosomes of the body and their supply to perforator flaps. Clin Plast Surg 2003;30:331-42. v. [Crossref] [PubMed]
- Early and locally advanced breast cancer: diagnosis and treatment. NICE guidance 2017.
- Burhenne HJ, Burhenne LW, Goldberg F, et al. Interval breast cancers in the Screening Mammography Program of British Columbia: analysis and classification. AJR Am J Roentgenol 1994;162:1067-71; discussion 72-5. [Crossref] [PubMed]
- Roy PG, Tenovici AA. Staged approach to partial breast reconstruction to avoid mastectomy in women with breast cancer. Gland Surg 2017;6:336-42. [Crossref] [PubMed]
- Chansakul T, Lai KC, Slanetz PJ. The postconservation breast: part 1, Expected imaging findings. AJR Am J Roentgenol 2012;198:321-30. [Crossref] [PubMed]
- Ott OJ, Schulz-Wendtland R, Uter W, et al. Fat necrosis after conserving surgery and interstitial brachytherapy and/or external-beam irradiation in women with breast cancer. Strahlenther Onkol 2005;181:638-44. [Crossref] [PubMed]
- Lövey K, Fodor J, Major T, et al. Fat necrosis after partial-breast irradiation with brachytherapy or electron irradiation versus standard whole-breast radiotherapy--4-year results of a randomized trial. Int J Radiat Oncol Biol Phys 2007;69:724-31. [Crossref] [PubMed]
- Ashkanani F, Sarkar T, Needham G, et al. What is achieved by mammographic surveillance after breast conservation treatment for breast cancer? Am J Surg 2001;182:207-10. [Crossref] [PubMed]
Cite this article as: Hu J, Tenovici A, Parulekar V, Bhattacharyya M, Roy PG. The impact of partial breast reconstruction with lateral chest wall perforator flaps on post-operative cancer surveillance. Ann Breast Surg 2018;2:10.