Rotation advancement flap—a novel technique for breast conserving surgery in tumors of the upper lateral breast quadrant
Introduction
Focus on patient’s quality of life and advances in oncological treatment of breast cancer have dramatically changed the paradigm of surgical treatment of breast cancer. Contemporary breast surgery has to provide not only with a radical surgery with good margins, but also with the best aesthetic result possible. These principles have been successfully implemented in oncoplastic breast conserving surgery and numerous studies have shown oncological safety of these techniques and high patient satisfaction (1,2).
In the era of precise diagnostics and improved tumor mapping, oncoplastic techniques are considered as safe as the classical wide local excision and, therefore, become a standard approach in breast centers worldwide. A great number of surgical techniques have been recently described for tumors of different locations (3-6).
Methods
In the current paper we propose a novel modification of an oncoplastic technique for breast tumors located in the upper-lateral quadrant. The aim of this method is a complete preservation of breast size and shape by a full compensation of the defect after tumor removal. For this purpose two principles may be utilized—flap rotation and advancement. Rotation advancement flap is a modification where a wide rotation flap from the lateral part of the breast is used along with a tissue complex from the axillary region. The point of rotation is the nipple-areolar complex (NAC).
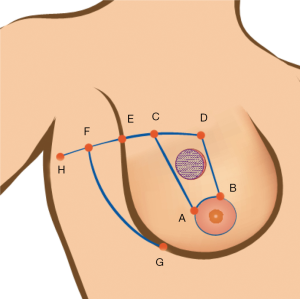
Preoperative markings are made according to the natural borders of the breast, lateral borders of mammary fold and areola. After the tumor borders are delineated, the area of removal is marked with two radial (or close to radial) lines along the upper medial (B, D) and lower lateral (A, C) borders of the tumor (Figure 1). The upper limit of the incision is marked horizontally, preferably according to the breast folds, and is extended to the axillary region along the curvature of the line DCEF. An area that equals the width of the area to be removed is marked on the line EF, from its intersection (point E) with the lateral mammary fold (EF = DC). In such way an upper lateral point is defined—upper border of a new lateral mammary fold. Finally, point F is smoothly joined with the lower lateral mammary fold level (G). Having done that, one has delineated the tumor area to be removed (A, B, D, C) as well as the lateral advancement flap (A, C, F, G) on a wide base. With this manoeuvre the removed area with the tumor (A, B, D, C) is compensated with the tissue from axillary region (G, F, E). Circumareolar mobilization of nipple-areola complex should be performed to minimize defects and changes of breast and areola shape.
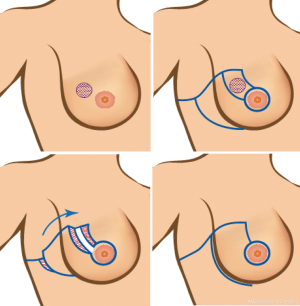
During the flap advancement, a rotation (not a linear tissue transfer) is performed with the NAC being the point of rotation (Figure 2). This principle is very close to a technique described in B-plasty (7). A wide mobilization of the parenchyma from the fascia is performed to obtain more natural position of the breast. It is crucial to identify and preserve the perforator vessels from aa. intercostales as well as the branches of a. thoracica lateralis (Figure 3) which should provide with an adequate blood supply to the flap and the rest of the parenchyma.
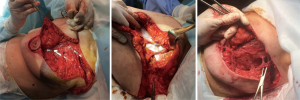
After a new position of NAC is defined (Figure 4), it is important to close the dead space with tight sutures in parenchyma, which protects the breast from unanticipated deformity, especially after radiotherapy. A round block mobilization of the nipple-areola complex is required to achieve a greater rotation. The wound closure is performed in a sitting position of the patient. An attention should be paid to a new lateral contour of the breast that has to correspond the natural position. This could be obtained by a wider mobilization (at GF level) and fixation to the chest wall as well as to the lateral part of the flap.
In some cases, an excessive tissue in the region of the distal edge of the wound (H point) should be excised. Axillary dissection usually does not affect operation planning or flap design, however, an extra effort should be taken to identify and preserve a. thoracica lateralis which is a flap supplying vessel.
Results
Between July 2013 and June 2017, 33 surgeries with the described technique were performed at the Breast Unit of LISOD Israeli Cancer Care Hospital. The mean age of the patients was 51 years (range, 33–69 years). Mean specimen weight was 113.5 g (range, 36–268 g), tumor size—26 mm (range, 10–50 mm). Six patients (18.2%) received neoadjuvant chemotherapy.
Sentinel lymph node biopsy (SLNB) was performed in 25 (75.8%) and axillary dissection in 12 (36.4%) patients. In non-palpable lesions (8/33, 24.2%), a fine needle localization with mammographic verification (n=5), radioguided occult lesion localization (n=2) or intraoperative ultrasound verification (n=1) were utilized. Involved margins were found in one (3.0%) case and the patient opted for a nipple-sparing mastectomy with immediate implant reconstruction. All patients but one received external beam postoperative radiotherapy in BED 50 Gr/25 fractions, 18 (54.5%) received adjuvant chemotherapy and 9 (27.3%) endocrine therapy.
Breast cancer stage distribution is presented in Table 1.
Table 1
| Stages | No. of patients (%) |
|---|---|
| 0 | 1 (3.0) |
| 1 | 6 (18.2) |
| 2a | 13 (39.4) |
| 2b | 7 (21.2) |
| 3a | 4 (12.2) |
| 3b | 1 (3.0) |
| 3c | 1 (3.0) |
Complications occurred in 7 (21.2%) patients: hematoma (n=2, including one requiring revision), cellulitis (n=3, conservative treatment), and wound edge necrosis (n=2, revision).
Notably, this technique was used in a patient after previous breast augmentation. With the breast implant preserved, a block of tissues from the axillary region was transferred to compensate the partial breast defect with an excellent aesthetic result (Figure 5).
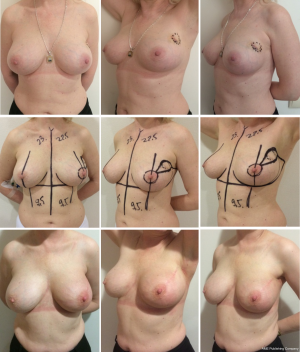
The proposed technique could be also utilized to correct secondary breast defects, e.g., following radiotherapy. Thus, one patient received a reconstruction following excision of a late (>2 years) radiation sequella (Figure 6). A marginal necrosis of the flap, however, was seen in the early postoperative period, which required revision surgery and debridement. The wound healed primary, but the late deformity of the gland was formed. We suggested the patient correction of the deformity with the symmetrizing reduction mammaplasty and lipografting, but the patient refused.
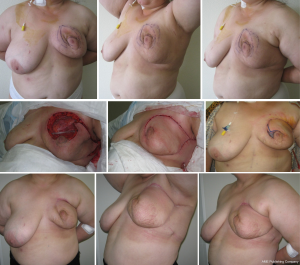
Thirty two (97%) patients were available for follow up, on average 29 (range, 6–48) months postoperatively. Follow up included clinical examinations every 3–4 months, ultrasound every 6 months and mammography every 12 months.
No loco-regional recurrence was registered, whereas distant metastases were revealed in 2 (6.1%) patients with clinical manifestations and confirmed with CT; 1 (3%) patient succumbed to breast cancer.
Discussion
The upper-lateral quadrant is one of most frequent locations of breast cancer (8-10). Partial mastectomy in this area is considered a relatively easy task for breast surgeons. Wide local excision with free margins with a good aesthetic result is achievable in cases of smaller tumors in large breast (C-D cup). Otherwise (i.e., larger tumor/smaller breast) it is often challenging to avoid a typical breast deformity such as deviation of the NAC and volume deficit of the lateral breast contour (Figure 7). It happens due to the loss of volume in the upper-lateral quadrant, which is typically compensated by the linear advancement of parenchyma of the lower part of the breast. This could often lead to NAC displacement from its geometric center and the volume of the gland inevitably decreases (Figure 7). Several methods have been proposed to prevent this distortion (Table 2).
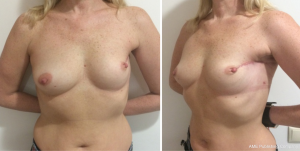
Table 2
| Procedure | Indications | Breast shape changes/symmetrizing procedure | Aesthetic results | Scheme of the surgery |
|---|---|---|---|---|
| Therapeutic mammaplasty using Wise pattern | For medium and large breasts, with ptosis (3–4 grade) | Significantly decreases the size of the breast, a symmetrizing contralateral operation is necessary | Excellent | |
| Tennis racquet | Applicable for any breast size and degree of ptosis, effective for smaller tumors | Moderately reduces the size of the breast, decreases volume in lateral quadrants, a symmetrizing procedure is recommended | Satisfactory | |
| Rotation flap | Effective for medium or larger breasts, with 1–2 degrees of ptosis | Moderately reduces the size of the breast, a symmetrizing operation is possible | Excellent | |
| Lateral thoracic flap | Applicable for any size of the breast and degree of ptosis | Minimally changes the size of the breast, may lead to the deformity. The symmetrizing procedure usually is not required. | Good to satisfactory |
Inferior flap mammaplasty, or a modified Ribeiro technique allows achieving a very good aesthetic result (11). However, both shape and size of the breast may change significantly, which frequently requires a symmetrizing procedure. In our practice, the patient’s anxiety for the cancer operation is often greater than the concerns regarding the aesthetic results, so patients are reluctant to/postponing symmetrizing procedures. Based on this, the methods of choice at our institution are the rotational flap and the lateral thoracical flap.
When using a rotational flap, a sector of the parenchyma with the tumor is removed to the full depth—from the skin and to the fascia of the m. pectoralis major. Then, the parenchyma is mobilized in the retromammary space, with the cutting of the skin along the submammary fold. It is also required to mobilize the NAC with a circular cutting of the dermis around the areola. Thus, we form a rotational flap from the lower sector of the gland and advance it to close the defect around the nipple. Nipple is the point of rotation. This maneuver preserves the shape, prevents deformations of the lateral part of the breast and ensures the full filling of the parenchymal defect. Nevertheless, the volume of the breast decreases, because the tissue of the gland is redistributed (Figure 8).
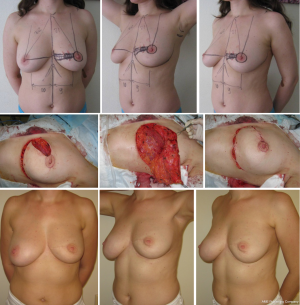
The lateral thoracic flap is an effective and useful method to address the defects in the lateral part of the breast. Holmström et al. initially suggested this technique as a simple method for implant coverage and volume deficits in delayed reconstruction (12,13). Munhoz et al. described this technique to close defects of the lateral sector of the breast in oncoplastic breast-conserving surgery (14). A similar technique was later published by Koh et al. where a skin-fascial flap from the axillary region on a wide base was used to fill the defect after tumor removal (15). This region has no aesthetic significance, and the quality of the skin and subcutaneous tissue here are very similar to those in the mammary gland (Figures 9,10).
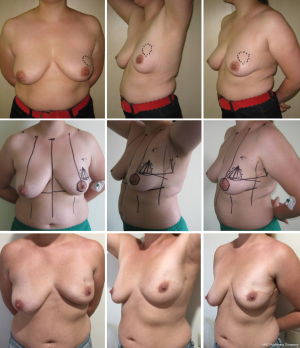
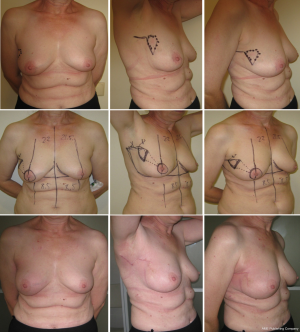
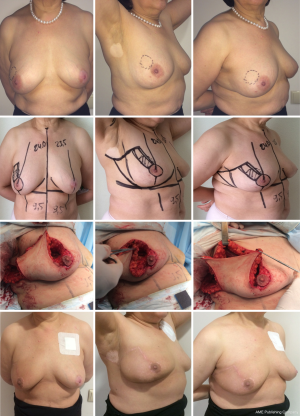
The rotation advancement flap may help to achieve a better aesthetic result, because it differs from the previously discussed techniques by using the natural boundaries of the gland and natural point of rotation (NAC). This technique can be effectively used for tumor located in upper-lateral quadrant of the breast, for different sizes of the breast—small (Figures 11,12), medium (Figure 13) and large (Figures 14,15). Sometimes it allows removing the bigger amount of tissue, e.g., in case of local recurrence (Figure 16). The following case is also noteworthy because it allows to compare different techniques—previously done wide local excision on the left side and our type of surgery on the right side.
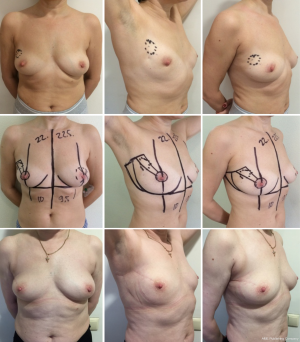
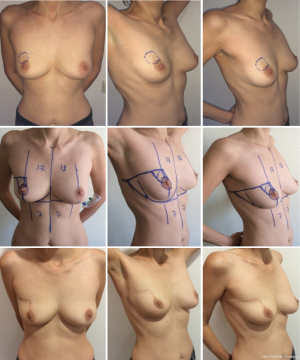
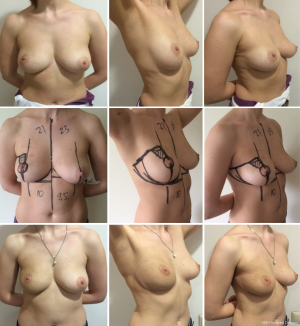

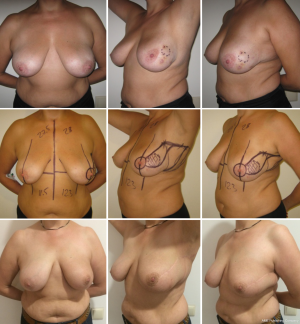
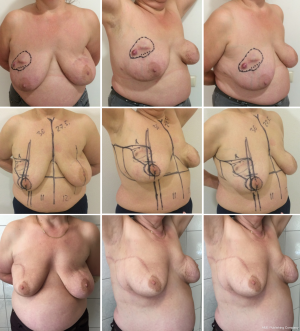
It is efficient even when the tumor is located in the upper sector of the breast (Figure 17).
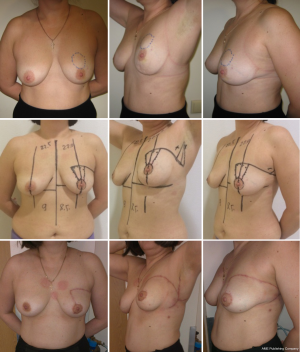
The complexity of this procedure is intermediate. It could be done by surgeons familiar with the therapeutic mammaplasty but not with island perforator flaps, such as LICAP or TDAP. Due to the wide base of the flap, a total flap necrosis is unlikely but partial marginal necrosis may occur due to aggressive tissue mobilization with the damage of the perforator vessels. We consider the proposed method more reliable and safe compared to the perforator island flap techniques, a consideration for younger surgeons. On the other hand, this technique has several disadvantages. These include the visible scars in the upper outer part of the breast (yet not in the décolleté area). Further, in larger tumors or in those located below the NAC level, this technique may cause a breast deformity (such as shown on Figure 14), which, however, can be corrected by fat grafting. Finally, like in any other type of breast conserving surgery, there are some reservations for the postradiation changes in the breast shape after rotation advancement flap procedure.
Our first experience with this method has been previously presented at the ESSO congresses (16,17) and the ORBS meeting (18).
Conclusions
We have proposed a novel technique of the rotation advancement flap in breast conserving surgery. This technique is reliable and allows correcting the deformities and significant volume loss. It may provide with an excellent aesthetic result and is considered a safe alternative (transition procedure) to more complex operations as island perforator flaps.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/abs.2018.01.02). AZ reports in addition, AZ has a patent (19) UA (11) 119416 (13) U (51) МПК (2017.01) A61B 17/00 issued. VP reports in addition, VP has a patent (19) UA (11) 119416 (13) U (51) МПК (2017.01) A61B 17/00 issued. DU has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This is a retrospective analysis and evaluation of the novel modification of a surgical technique to reconstruct the breast after tumor removal during breast conserving surgery. All study participants signed the preoperative informed consent. The article was approved by the ethical committee of the LISOD Hospital of Israeli Oncology, where all the surgeries have been performed.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Agarwal S, Pappas L, Neumayer L, et al. Effect of breast conservation therapy vs mastectomy on disease-specific survival for early-stage breast cancer. JAMA Surg 2014;149:267-74. [Crossref] [PubMed]
- Ho A, Morrow M. The evolution of the locoregional therapy of breast cancer. Oncologist 2011;16:1367-79. [Crossref] [PubMed]
- Losken A, Dugal CS, Styblo TM, et al. A meta-analysis comparing breast conservation therapy alone to the oncoplastic technique. Ann Plast Surg 2014;72:145-9. [Crossref] [PubMed]
- Clough KB, Kaufman GJ, Nos C, et al. Improving breast cancer surgery: a classification and quadrant per quadrant atlas for oncoplastic surgery. Ann Surg Oncol 2010;17:1375-91. [Crossref] [PubMed]
- McCulley SJ, Macmillan RD. Planning and use of therapeutic mammoplasty--Nottingham approach. Br J Plast Surg 2005;58:889-901. [Crossref] [PubMed]
- Clough KB, van la Parra RFD, Thygesen HH, et al. Long-term Results After Oncoplastic Surgery for Breast Cancer: A 10-year Follow-up. Ann Surg 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Fitzal PS, Schrenk P. Oncoplastic breast surgery. Springer, 2010.
- Fitoussi AD, Berry MG, Famà F, et al. Oncoplastic breast surgery for cancer: analysis of 540 consecutive cases Plast Reconstr Surg 2010;125:454-62. [outcomes article]. [Crossref] [PubMed]
- Schaverien MV, Raine C, Majdak-Paredes E, et al. Therapeutic mammaplasty--extending indications and achieving low incomplete excision rates. Eur J Surg Oncol 2013;39:329-33. [Crossref] [PubMed]
- Fowble B, Solin LJ, Schultz DJ, et al. Breast recurrence and survival related to primary tumor location in patients undergoing conservative surgery and radiation for early-stage breast cancer. Int J Radiat Oncol Biol Phys 1992;23:933-9. [Crossref] [PubMed]
- Ribeiro L, Accorsi A Jr, Buss A, et al. Creation and evolution of 30 years of the inferior pedicle in reduction mammaplasties. Plast Reconstr Surg 2002;110:960-70. [Crossref] [PubMed]
- Blomqvist L, Malm M. Clinical experience with the lateral thoracodorsal flap in breast reconstruction. Ann Plast Surg 1999;43:7-13. [Crossref] [PubMed]
- Blomqvist L, Malm M, Holmström H, et al. The lateral thoracodorsal flap in breast reconstruction: a comparison between two plastic surgical centres. Scand J Plast Reconstr Surg Hand Surg 2000;34:327-30. [Crossref] [PubMed]
- Munhoz AM, Montag E, Arruda EG, et al. The role of the lateral thoracodorsal fasciocutaneous flap in immediate conservative breast surgery reconstruction. Plast Reconstr Surg 2006;117:1699-710. [Crossref] [PubMed]
- Koh SH, Seo HI, Bae YT. Immediate conservative breast reconstruction technique using lateral thoracodorsal fasciocutaneous Flap. J Breast Cancer 2007;10:217-22. [Crossref]
- Zhygulin A, Palitsa V, Dmytrenko O. 196. Extended rotational flap for closing defects of upper-lateral segment of the breast. The useful trick in oncoplastic breast conserving surgery. Eur J Surg Oncol 2014;40:S83-S84. [Crossref]
- Zhygulin A, Palytsia V, Dmytrenko O, et al. 92. Oncoplastic techniques in upper-lateral tumor location: Development of classical and introduction of modern techniques. Eur J Surg Oncol 2016;42:S104. [Crossref]
- Available online: http://www.orbsweb.com/orbs-content/recent-content/2015/2015-abstracts-posters/oncoplastic-breast-conserving-surgery-in-central-tumors-oncological-and-technical-aspects/
Cite this article as: Zhygulin A, Palytsia V, Unukovych D. Rotation advancement flap—a novel technique for breast conserving surgery in tumors of the upper lateral breast quadrant. Ann Breast Surg 2018;2:4.